Pharmaceuticals Management
The pharmaceutical and medical sectors are renowned for their complexity and scale of regulation. High national and international standards for quality, safety and process accuracy reflect the acute responsibility the sectors hold for patients; the consequences of getting it wrong can be disastrous, including the loss of life.
The pharmaceutical sector is a global industry today with worldwide supply chains, manufacturing networks and markets. This leads to more legislative control emerging to ensure patient safety as complexity grows and visibility of supply chains deteriorates.
The GS1 System of Standards has been established to improve supply chain efficiencies in numerous sectors, including pharmaceuticals. The use of the standard in healthcare is designed to increase patient safety, drive supply chain efficiency and improve, in particular, the traceability of medicines.
SATO, as a leading label and printing technology provider to the healthcare sector, offers a range of GS1 compliant barcode solutions to meet the exacting needs of this important industry. GS1 barcodes and standards help to address the foundational challenges healthcare providers face and deliver greatly improved patient safety and cost-saving operational efficiencies.
Serialisation & label validation

In Europe, the EU Falsified Medicine Directive (FMD) came into play for all EU member states in February 2016 to fully harmonise the use of unique identifiers across the EU.
The directive ensures that safety features for medicine, pharmaceutical and medical device packaging are incorporated onto labels and packaging by February 2019 including data matrices on secondary level packaging as well as unique identification numbers, batch/lot numbers, expiry dates, serial numbers and national reimbursement numbers, if applicable.
To address this, SATO offers various ways to easily inspect printed labels, ranging from a simple barcode verification system provided by Datalogic and available on both CL4NX Plusand CL6NX series, to a full label verification including OCR (optical character recognition) and OCV (optical character verification) inspection available on the CL4NX Plusseries.
To comply with the directive, a datamatrix code containing the following information must be applied to all medicine packaging by 2019:
- Incorporate a sequence of numeric or alphanumeric characters that is unique to a given pack of a medicinal product
- The code allows the identification of at least the name, the common name, the pharmaceutical form, the strength, the pack size and the pack type of the medicinal product bearing the unique identifier (‘product code’)
- The code is a numeric or alphanumeric sequence of maximum 20 characters, generated by a deterministic or a non-deterministic randomisation algorithm (‘serial number’)
- A national reimbursement number or other national number identifying the medicinal product, if required by the Member State where the product is intended to be placed on the market; 9.2.2016 L 32/7 Official Journal of the European Union EN
- The batch number
- The expiry date
- A tamper-evident device, such as a seal, must also be applied
Find out more GS1 relevant information for Healthcare providers
See SATO Solutions
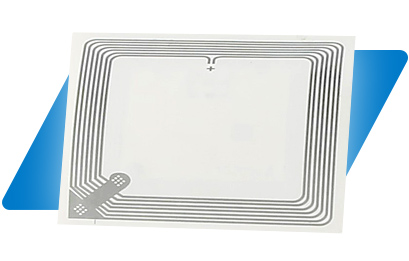
RFID controlled drugs safety

By automating the inventory management process in healthcare organisations large or small, the need for labour-intensive manual processes can be reduced and the likelihood of human errors minimised reducing costly mistakes.
View our New Ways of Managing Inventory section for more information.
See SATO Solutions
Contact us